In 2005, Bristol High School students began a series of investigations into the iron ion content of our local watershed; the Otter Creek. Approximately 1.9% of the Earth’s crust is iron. In nature, iron is found as two types of ions: Fe2+ and Fe3+. The students knew that the cycling between these two states of iron ions has a significant effect on both the living and non-living parts of the freshwater environment.
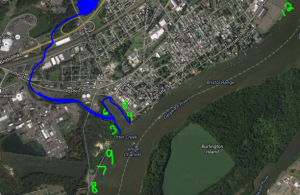
Arial photo of the Otter Creek Watershed
Together with their teacher, Dr. Bill Smith, they set out to investigate the pattern of this cycling in Otter Creek, Magnolia Lake, Silver Lake, and the Bristol Marsh; all parts of the same watershed.

Using the Absorption Spectrometer
Initial investigations focused on Fe2+ concentrations. During the first years of the project, the students used Villanova University’s Atomic Absorption Spectrometer. After a $10,000 grant from Toyota Motor Sales, USA and generous support from Dow and the National Science Foundation, the students were able to purchase an Ultraviolet/Visible light spectrometer for Bristol High School and continue the research on site.
Award winning research results
Each year, the students published their research in the form of a poster that was displayed at an annual poster session held by the Philadelphia Section of the American Chemical Society. Four of the posters won awards. Most notable is that the group discovered a new method for determining Fe3+ concentrations in natural waters. This research is scheduled to be published in the Journal of Chemical Education, the world’s premier journal focusing on university chemical education, in February 2020.
